Discovery and mechanistic investigation of Pt-catalyzed oxidative homocoupling of benzene with PhI(OAc)2†
Abstract
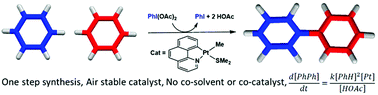
We present a Pt-catalyzed direct coupling of benzene to biphenyl. This catalytic reaction employs a cyclometalated platinum(II) complex [PtMe(bhq)(SMe2)] (bhq = benzo[h]quinolate) with PhI(OAc)2 as an oxidant and does not require an acid, a co-catalyst or a solvent. The reaction kinetics and characterization of potential catalytic species are reported. The reaction is first-order in Pt and second-order in benzene, which implicates the second C–H activation step as rate-determining. A Pt(II)/Pt(IV) catalytic cycle is suggested. The reaction commences by oxidation of the Pt(II) complex to give the platinum(IV) species [Pt(bhq)(SMe2)(OAc)2](OAc) followed by C–H activation of benzene to afford the intermediate [PtPh(bhq)(SMe2)(OAc)](OAc) concurrently with the release of HOAc. A second benzene molecule reacts similarly to give the diphenyl intermediate [PtPh2(bhq)(SMe2)](OAc). C–C bond forming reductive elimination ensues to regenerate Pt(II) and complete the catalytic cycle. The proposed mechanism has been examined by DFT computations, which provide support to experimental findings.
- This article is part of the themed collection: Inorganic Reaction Mechanisms


 Please wait while we load your content...
Please wait while we load your content...