γ-Radiation and H2O2 induced oxidative dissolution of uranium(iv) oxide in aqueous solution containing phthalic acid
Abstract
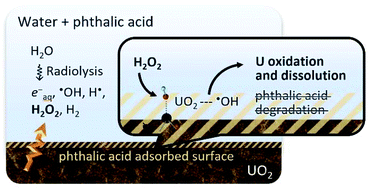
This study aims to reveal possible involvements of organic acids in the oxidative dissolution of UO2. Using phthalic acid as a model compound, we have measured adsorption on UO2 and investigated effects on the reaction between H2O2 and UO2 and on oxidative dissolution induced by γ-irradiation. Significant adsorption of phthalic acid was observed even at neutral pH. However, the reaction between H2O2 and UO2 in phthalic acid solution induced oxidative dissolution of U(VI) similar to in aqueous bicarbonate solution. Moreover, degradation products of phthalic acid were not detected after the reaction of H2O2. These results indicate that even though phthalic acid adsorbs on the UO2 surface, it is not involved in the interfacial reaction of H2O2. In contrast, the dissolution of U by irradiation was inhibited in aqueous phthalic acid solution, whereas H2O2 generated by radiolysis was consumed by UO2. Based on these contrasting results, possible roles of radical species generated by water radiolysis were discussed.


 Please wait while we load your content...
Please wait while we load your content...