Open-framework ammonium transition metal fluorophosphates with a Kagomé lattice network: synthesis, structure and magnetic properties†
Abstract
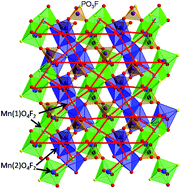
Two open-framework transition metal fluorophosphates (NH4)M3(PO3F)2(PO2F2)F2 (M = Mn and Co) have been synthesized under fluoride-rich hydrothermal conditions at 493 K. The structure consists of triplets of face-sharing trans-MO4F2 octahedra linked by PO3F groups to form 2D layers. These 2D layers with formula [M3(PO3F)2O2F2]3− in the ab plane are bridged by PO2F2 groups to generate a 3D open framework with 1D channels filled with ammonium cations. The XPS spectra suggested the existence of Mn(II) and Co(II) in the corresponding compounds. Further, the XPS F 1s spectra indicated that fluorine anions bridged both the M and P centres. The two compounds feature transition metal layers with a staircase Kagomé network. This topology promotes magnetic frustration, which exhibits a canted antiferromagnetic ground state (Tc = 8.0 K for Mn and Tc = 9.2 K for Co).


 Please wait while we load your content...
Please wait while we load your content...