Ligand-free, catalytic and regioselective hydroboration of selenoalkynes†
Abstract
The copper-catalyzed hydroboration of selenoalkynes in a regio- and stereoselective fashion is reported, delivering selenium-containing vinylboronate products in good yields. The reported protocol fills an important gap in the literature with respect to the synthesis of a valuable class of compounds, which is difficult to obtain otherwise.
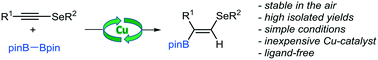


 Please wait while we load your content...
Please wait while we load your content...