Palladium-catalyzed cross-couplings by C–O bond activation
Abstract
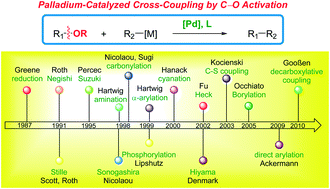
Although palladium-catalyzed cross-coupling of aryl halides and reactive pseudohalides has revolutionized the way organic molecules are constructed today across various fields of chemistry, comparatively less progress has been made in the palladium-catalyzed cross-coupling of less reactive C–O electrophiles. This is despite the fact that the use of phenols and phenol derivatives as bench-stable cross-coupling partners has been well-recognized to bring about major advantages over aryl halides, such as natural abundance of phenols, (2) avoidance of toxic halides, (3) orthogonal cross-coupling conditions, (4) prefunctionalization of phenolic substrates by electrophilic substitution or C–H functionalization, (5) ready availability of phenols from a different pool of precursors than aryl halides. In this review, we present an overview of recent advances made in the field of palladium-catalyzed cross-coupling of C–O electrophiles with a focus on catalytic systems, (2) reaction type, and (3) class of C–O coupling partners. Although the field has been historically dominated by nickel catalysis, it is now evident that the use of more versatile, more functional group tolerant and highly active palladium catalysts supported by appropriately designed ancillary ligands enables the cross-coupling with improved substrate scope and generality, and likely represents a practical solution to the broadly applicable cross-coupling of various C–O bonds across diverse chemical disciplines. The review covers the period through June 2020.


 Please wait while we load your content...
Please wait while we load your content...