Electrocatalytic oxygen and hydrogen evolution reactions at Ni3B/Fe2O3 nanotube arrays under visible light radiation†
Abstract
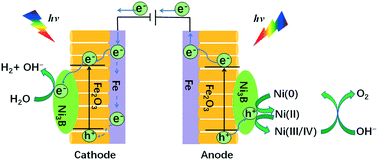
We demonstrate that arrays of Ni3B/Fe2O3 nanotubes (NTAs) supported by a Fe foil can simultaneously boost the kinetics of the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) after exposure to visible light radiation (OER and HER overpotentials decreased by 162 and 150 mV at 10 mA cm−2, respectively). With a small band gap, Fe2O3 efficiently harnesses solar energy for the overall photoelectrochemical water splitting. Consequently, a current density of 10 mA cm−2 requires only 1.50 V under one-sun illumination. Surface and spectroscopic analyses indicate that photoholes at the Fe2O3 NTAs transfer to Ni3B, rendering the Ni3B surface easier to oxidize into β-Ni(OH)2, which is ultimately converted into the OER-catalyzing γ-NiOOH species at a relatively low anodic potential. Photoelectrons become localized at the HER-active Ni sites in Ni3B. This work integrates photochemistry with electrocatalysis, affording a new avenue for developing high-performance and cost-effective photoelectrocatalysts for energy conversion applications.


 Please wait while we load your content...
Please wait while we load your content...