Intertwined chemistry of hydroxyl hydrogen-bond donors, epoxides and isocyanates in the organocatalytic synthesis of oxazolidinones versus isocyanurates: rational catalytic investigation and mechanistic understanding†
Abstract
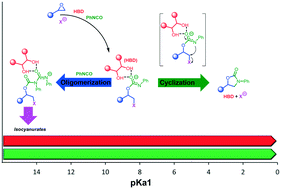
Hydroxyl compounds represent an important family of organocatalysts with the ability to coordinate and activate reaction substrates by establishing an array of hydrogen bonds. Thus far, the use of hydroxyl hydrogen bond donors (HBDs) has been scarcely investigated in the cycloaddition of isocyanates to epoxides to afford 3-aryl-2-oxazolidinones as a class of heterocycles with promising applications in the pharmaceutical chemistry. In this work, we carried out a systematic investigation of readily available HBDs as catalytic components of binary systems for the latter cycloaddition reaction. Such a study showed the crucial role of the HBDs' pKa1 value in driving the selectivity of the reaction towards oxazolidinones as opposed to the formation of oligomeric isocyanurates or ureas, with the most acidic HBDs (pKa1: 3–4) displaying the best catalytic performance. Mechanistic investigation with the support of DFT calculations allowed deeper insight into the reaction dynamics and the origin of the experimentally observed chemoselectivity for cyclization versus cyclo-oligomerization at different reaction temperatures, shedding light on a strictly intertwined chemistry between the HBDs, isocyanates and epoxides.


 Please wait while we load your content...
Please wait while we load your content...