Synergistic effect on BCN nanomaterials for the oxygen reduction reaction – a kinetic and mechanistic analysis to explore the active sites†
Abstract
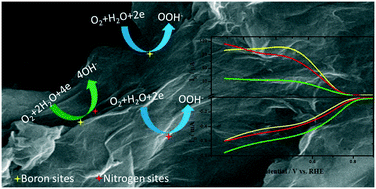
Metal-free heteroatom-doped carbon nanomaterials are promising alternatives to high-cost state-of-the-art platinum catalysts and less stable second-generation non-platinum group metal oxygen reduction reaction (ORR) catalysts. Literature reports on the ORR activity of metal-free catalysts have mostly focused on nitrogen-doped carbon materials. Recent studies demonstrate that co-doping of a carbon material with two heteroatoms often shows higher electrocatalytic activity than when a carbon material is doped with either of the two individual heteroatoms. Co-doping of carbon materials with B and N has already been studied, and the increase in catalytic activity can be explained by the synergistic effect arising from B and N. Here, we report B- and N-doped thermally reduced graphene oxide (B&N-rGO) made from previously synthesised B-doped rGO and N-doped rGO. The B&N-rGO, when compared with B-rGO and N-rGO, shows a positive shift in onset potential and predominantly follows the four-electron ORR pathway. The kinetic rate constants, estimated from the Damjanovic model, demonstrate that the change in the mechanistic pathway is mainly due to the four-electron active sites made-up of B and N. The mechanism of the synergistic effect was probed using a kinetic analysis conducted at various loading densities. The dissociative electron transfer pathway of the ORR on B&N-rGO indicates that O2 simultaneously binds to boron and carbon near to the nitrogen active sites.


 Please wait while we load your content...
Please wait while we load your content...