Catalytic hydration of cyanamides with phosphinous acid-based ruthenium(ii) and osmium(ii) complexes: scope and mechanistic insights†
Abstract
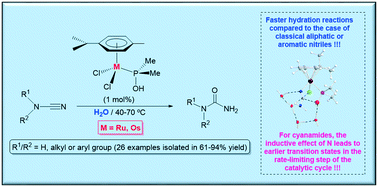
The synthesis of a large variety of ureas R1R2NC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)NH2 (R1 and R2 = alkyl, aryl or H; 26 examples) was successfully accomplished by hydration of the corresponding cyanamides R1R2NC
O)NH2 (R1 and R2 = alkyl, aryl or H; 26 examples) was successfully accomplished by hydration of the corresponding cyanamides R1R2NC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N using the phosphinous acid-based complexes [MCl2(η6-p-cymene)(PMe2OH)] (M = Ru (1), Os (2)) as catalysts. The reactions proceeded cleanly under mild conditions (40–70 °C), in the absence of any additive, employing low metal loadings (1 mol%) and water as the sole solvent. In almost all the cases, the osmium complex 2 featured a superior reactivity in comparison to that of its ruthenium counterpart 1. In addition, for both catalysts, the reaction rates observed for the hydration of the cyanamide substrates were remarkably faster than those involving classical aliphatic and aromatic nitriles. Computational studies allowed us to rationalize all these trends. Thus, the calculations indicated that the presence of a nitrogen atom directly linked to the C
N using the phosphinous acid-based complexes [MCl2(η6-p-cymene)(PMe2OH)] (M = Ru (1), Os (2)) as catalysts. The reactions proceeded cleanly under mild conditions (40–70 °C), in the absence of any additive, employing low metal loadings (1 mol%) and water as the sole solvent. In almost all the cases, the osmium complex 2 featured a superior reactivity in comparison to that of its ruthenium counterpart 1. In addition, for both catalysts, the reaction rates observed for the hydration of the cyanamide substrates were remarkably faster than those involving classical aliphatic and aromatic nitriles. Computational studies allowed us to rationalize all these trends. Thus, the calculations indicated that the presence of a nitrogen atom directly linked to the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond depopulates electronically the nitrile carbon by inductive effect when coordinated to the metal center, thus favouring the intramolecular nucleophilic attack of the OH group of the phosphinous acid ligand to this carbon. On the other hand, the higher reactivity of Os vs. Ru seems to be related with the lower ring strain on the incipient metallacycle that starts to form in the transition state associated with this key step in the catalytic cycle. Indirect experimental evidence of the generation of the metallacyclic intermediates was obtained by studying the reactivity of [RuCl2(η6-p-cymene)(PMe2OH)] (1) towards dimethylcyanamide in methanol and ethanol. The reactions afforded compounds [RuCl(η6-p-cymene)(PMe2OR)(N
N bond depopulates electronically the nitrile carbon by inductive effect when coordinated to the metal center, thus favouring the intramolecular nucleophilic attack of the OH group of the phosphinous acid ligand to this carbon. On the other hand, the higher reactivity of Os vs. Ru seems to be related with the lower ring strain on the incipient metallacycle that starts to form in the transition state associated with this key step in the catalytic cycle. Indirect experimental evidence of the generation of the metallacyclic intermediates was obtained by studying the reactivity of [RuCl2(η6-p-cymene)(PMe2OH)] (1) towards dimethylcyanamide in methanol and ethanol. The reactions afforded compounds [RuCl(η6-p-cymene)(PMe2OR)(N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CNMe2)][SbF6] (R = Me (5a), Et (5b)), resulting from the alcoholysis of the metallacycle, which could be characterized by single-crystal X-ray diffraction.
CNMe2)][SbF6] (R = Me (5a), Et (5b)), resulting from the alcoholysis of the metallacycle, which could be characterized by single-crystal X-ray diffraction.


 Please wait while we load your content...
Please wait while we load your content...