Hydrodeoxygenation of anisole to benzene over an Fe2P catalyst by a direct deoxygenation pathway†
Abstract
Developing economically viable and highly selective hydrodeoxygenation (HDO) catalysts for the C–O bond cleavage of lignin-derived aryl ethers is very important, but still challenging. Herein, a novel monometallic Fe2P catalyst was synthesized by a two-stage phosphorization method under mild conditions, which showed a high arene (benzene) selectivity of 96.7% among C6+ products with a turnover frequency of 8.2 × 10−3 mol molCO−1 s−1 in the HDO of anisole at 200 °C and ambient hydrogen pressure. Characterization results revealed that the crystal structure of the Fe2P catalyst was stable and no phosphorus loss occurred in the reduction and reaction process. The kinetic results indicated that the coverage of anisole was higher than that of H2 on the Fe2P catalyst. The anisole temperature-programmed surface reaction (anisole-TPSR) and density functional theory (DFT) calculation results identified that the anisole HDO reaction mechanism is the direct cleavage of the Caryl–O bond of anisole to form 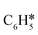 and CH3O* without forming the C6H5–OH–CH3 intermediate. These findings may contribute toward understanding the iron-based phosphide and guiding the catalyst design for the HDO of aryl ethers and related reactions.
and CH3O* without forming the C6H5–OH–CH3 intermediate. These findings may contribute toward understanding the iron-based phosphide and guiding the catalyst design for the HDO of aryl ethers and related reactions.



 Please wait while we load your content...
Please wait while we load your content...