The catalytic combustion of CH2Cl2 over SO42−–TixSn1−x modified with Ru†
Abstract
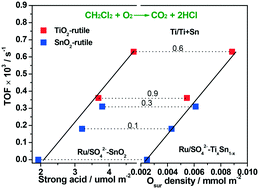
Ru/SO42−–TixSn1−x catalysts were prepared via impregnation with an aqueous solution of RuCl3 on SO42−–TixSn1−x. For the samples with a Ti/Ti + Sn ratio higher or lower than 0.6, TiO2 or SnO2-rutile existed, and both contained the Ti–O–Sn structure. The residual SO42− species as chelating or bridged bidentate complexes with the Ti4+ and Sn4+ ions promoted the acidity significantly. Ru as Ru0 and Ru4+ was highly dispersed on the matrices as a result of the interaction between the Ru and SO42− species. Ti–O–Sn increased the surface oxygen and SO42− complexes, while Ru promoted the redox activity. In CH2Cl2 oxidation, the Ru/SO42−–TixSn1−x catalysts with the Ti–O–Sn structure presented high activity with TOFs at 225 °C of 0.18–0.63 × 10−3 s−1, which could be ascribed to the synergism between SO42−–Ti4+ and the active surface oxygen. At 90% conversion, the selectivity for CO2 and HCl was 90% or higher. Furthermore, the Ru/SO42−–TixSn1−x catalysts maintained stable activity for 50 h in wet or dry feed without significant changes. The in situ FT-IR spectra showed that the –O–CH2–O– organic sulfate was the main intermediate in CH2Cl2 oxidation. The hydroxyl activated by surface oxygen was responsible for high activity and Cl removal.


 Please wait while we load your content...
Please wait while we load your content...