Tutorial review on structure – dendrite growth relations in metal battery anode supports
Abstract
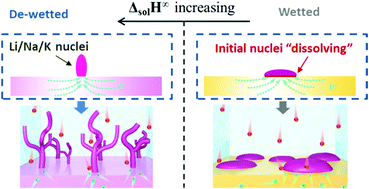
This tutorial review explains the emerging understanding of the surface and bulk chemistry – electrochemical performance relations in anode supports (aka secondary current collectors, substrates, templates, hosts) for lithium, sodium and potassium metal batteries (LMBs, SMBs or NMBs, and KMBs or PMBs). In relation to each section, the possible future research directions that may yield both new insight and improved cycling behavior are explored. Representative case studies from Li, Na and K metal anode literature are discussed. The tutorial starts with an overview of the solid electrolyte interphase (SEI), covering both the “classic” understanding of the SEI structure and the “modern” insights obtained by site-specific cryogenic stage TEM analysis. Next, the multiple roles of supports in promoting cycling stability are detailed. Without an optimized support architecture, the metal–electrolyte interface becomes geometrically unstable at a lower current density and cycle number. Taking into consideration the available literature on LMBs, SMBs and KMBs, it is concluded that effective architectures are geometrically complex and electrochemically lithiophilic, sodiophilic or potassiophilic, so as to promote conformal electrochemical wetting of the metal during plating/stripping. One way that philicity is achieved is through support oxygen surface chemistry, which yields a reversibly reactive metal–support interface. Examples of this include the well-known oxygen–carbon moieties in reduced graphene oxide (rGO), as well as classic ion battery reversible conversion reaction oxides such as SnO2. Unreactive surfaces lead to dewetted island growth of the metal, which is a precursor to dendrites, and possibly to non-uniform dissolution. Surveying the literature on various Li, Na and K metal supports, it is concluded that the key bulk thermodynamic property that will predict electrochemical wetting behavior is the enthalpy of infinite solution (ΔsolH∞) of the metal (solute) into the support (solvent). Large and negative ΔsolH∞ promotes uniform metal wetting on the support surface, corresponding to relatively low plating overpotential. Positive ΔsolH∞ promotes dewetted islands and a relatively high overpotential. This simple rule explains a broad range of studies on Li, Na and K metal – support interactions, including the previously reported correlation between mutual solubility and wetting.


 Please wait while we load your content...
Please wait while we load your content...