Formazanate coordination compounds: synthesis, reactivity, and applications
Abstract
Formazans (Ar1-NH-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR3-N
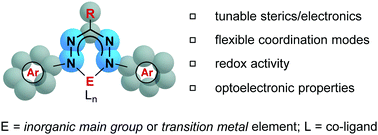
CR3-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-Ar5), a class of nitrogen-rich and highly colored compounds, have been known since the late 1800s and studied more closely since the early 1940s. Their intense color has led to their widespread use as dyes, especially in cell biology where they are most often used to quantitatively assess cell-viability. Despite structural similarities to well-known ligand classes such as β-diketiminates, the deprotonated form of formazans, formazanates, have received relatively little attention in the transition metal and main group coordination chemistry arenas. Formazanate ligands benefit from tunable properties via structural variation, rich optoelectronic properties owing to their highly delocalized π-systems, low-lying frontier orbitals that stabilize otherwise highly reactive species such as radicals, and redox activity and coordinative flexibility that may have significant implications in their future use in catalysis. Here, we review progress in the coordination chemistry of formazanate ligands over the past two decades, with emphasis on the reactivity and applications of the subsequent complexes.
N-Ar5), a class of nitrogen-rich and highly colored compounds, have been known since the late 1800s and studied more closely since the early 1940s. Their intense color has led to their widespread use as dyes, especially in cell biology where they are most often used to quantitatively assess cell-viability. Despite structural similarities to well-known ligand classes such as β-diketiminates, the deprotonated form of formazans, formazanates, have received relatively little attention in the transition metal and main group coordination chemistry arenas. Formazanate ligands benefit from tunable properties via structural variation, rich optoelectronic properties owing to their highly delocalized π-systems, low-lying frontier orbitals that stabilize otherwise highly reactive species such as radicals, and redox activity and coordinative flexibility that may have significant implications in their future use in catalysis. Here, we review progress in the coordination chemistry of formazanate ligands over the past two decades, with emphasis on the reactivity and applications of the subsequent complexes.


 Please wait while we load your content...
Please wait while we load your content...