Unravelling the electronic nature of C–F⋯O–C non-covalent interaction in proteins and small molecules in the solid state†‡
Abstract
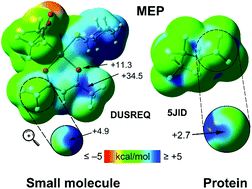
The participation of organic fluorine as a halogen bond donor is rare and is sensitive to the electronic environment in the vicinity of the fluorine atom. The enhancement in the electropositive character (the σ-hole formalism) in fluorine is established by the presence of electron withdrawing groups and this has been examined in the solid-state structures in small molecules and proteins. Short, directional F⋯O contacts have been observed and physical insights obtained, from quantum mechanical calculations, via the molecular electrostatic potential, an analysis of their topological features from atoms-in-molecules, and donor–acceptor characteristics from natural bond orbital analyses. It was observed that such contacts, cooperatively act in the presence of other interactions, and the formed aggregates are stabilizing in nature. In addition, the F⋯O has a bonding character and is attractive in nature. The halogen bonding character of fluorine is relevant in supramolecular chemistry.


 Please wait while we load your content...
Please wait while we load your content...