A quantum-based molecular dynamics study of the ICM-102/HNO3 host–guest reaction at high temperatures†
Abstract
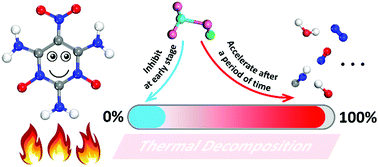
The contradiction between energy and safety of explosives is better balanced by the host–guest inclusion strategy. Understanding the reaction mechanism of the host–guest explosive is necessary. To deeply analyze the role of the small guest molecules in the host–guest system, a quantum-based molecular dynamics method was used to calculate the initial decomposition reaction of the new host–guest explosive ICM-102/HNO3 against the pure ICM-102 at several high temperatures. The incorporation of HNO3 had no significant influence on the initial decomposition step of ICM-102. Conversely, the earliest intramolecular hydrogen transfer reaction is delayed partly because the H and O atoms of HNO3 connect with the O and H atoms of ICM-102, respectively. As the reaction proceeds, guest molecules get heavily involved in the reaction and increase the reaction rate. The generation rate and quantity of the small oxidizing molecules in the final product were increased significantly in the ICM-102/HNO3 system. These mechanisms revealed that HNO3 molecules inhibit the early stages of the initial decomposition of ICM-102 to some extent, and play an important role in accelerating the decomposition subsequently.


 Please wait while we load your content...
Please wait while we load your content...