UV-induced radical formation and isomerization of 4-methoxyindole and 5-methoxyindole†
Abstract
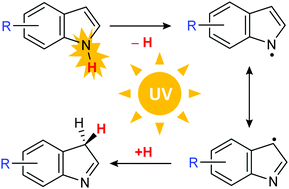
Monomers of 4-methoxyindole and 5-methoxyindole trapped in low-temperature xenon matrices (15–16 K) were characterized by IR spectroscopy, in separate experiments. Each compound was shown to adopt the most stable 1H-tautomeric form. The photochemistry of the matrix-isolated compounds was then investigated by exciting the matrices with narrowband UV light with λ ≤ 305 nm. Two main photoproducts, similar for each compound, have been detected: (1) 4-methoxy- or 5-methoxy-indolyl radical, resulting from cleavage of the N–H bond; (2) 3H-tautomers (4-methoxy- or 5-methoxy-) with the released hydrogen atom reconnected at the C3 ring carbon atom. The presence of the two types of photoproducts in the UV-irradiated matrices was confirmed by comparison of their B3LYP/6-311++G(d,p) calculated IR spectra with the experimental spectra emerging upon the irradiations. The mechanism of the observed phototransformations was elucidated by Natural Bond Orbital and Natural Resonance Theory computations on the methoxy-substituted indolyl radicals resulting from the N–H bond cleavage. The highest natural atomic spin densities were predicted at the C3 and N1 positions of the indolyl ring, corresponding to a predominance of the resonance structures with the radical centres located at these two atoms. As a whole, the obtained experimental and theoretical data allowed establishing a general pattern for the photochemistry of methoxyindoles under matrix-isolation conditions.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...