Crystal facet-dependent activity of h-WO3 for selective conversion of furfuryl alcohol to ethyl levulinate†
Abstract
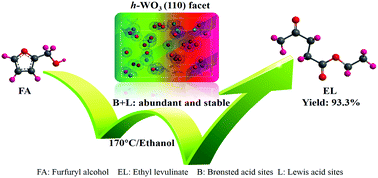
The use of WO3 as an acid catalyst has received extensive attention in recent years. However, the correlation between the catalytic activity and the predominantly exposed surface with varied acidic sites needs further understanding. Herein, the effects of the Brønsted and Lewis acid sites of different crystal facets of WO3 on the catalytic conversion of furfuryl alcohol (FA) to ethyl levulinate (EL) in ethanol were investigated in detail. A yield of EL up to 93.3% over WO3 with the (110) facet exposed was achieved at 170 °C, while FA was mainly converted to polymers over (001) faceted nanosheets and nanobelts with exposed (002) and (100) facets. This was attributed to the different distribution of the acidic sites on different exposed crystal facets. The (110) faceted WO3 possessed abundant and strong Brønsted acid sites, which favored the conversion of FA to EL, while the (100) faceted WO3 with stronger Lewis acid sites and weaker Brønsted acid sites mainly led to the formation of polymers. In addition, the (110) faceted WO3 showed excellent sustainability in comparison with the (100) faceted counterpart.


 Please wait while we load your content...
Please wait while we load your content...