Temperature- and pressure-dependent rate coefficient measurement for the reaction of CH2OO with CH3CH2CHO†
Abstract
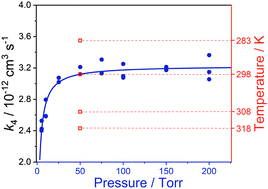
Propionaldehyde is one of the most abundant aldehydes, which are an important class of volatile organic compounds. In this work, the rate coefficient of the reaction of the simplest Criegee intermediate CH2OO with propionaldehyde (CH3CH2CHO) was measured for the first time in a flash photolysis reaction tube by using the OH laser-induced fluorescence (LIF) method at temperature and pressure in the range of 283 to 318 K and 5 to 200 Torr. This reaction is observed to be pressure- and temperature-dependent. The measured rate coefficient at 50 Torr is in the vicinity of the high-pressure limit value of (3.23 ± 0.49) × 10−12 cm3 s−1 at 298 K, which is in agreement with a previously reported theoretical result of 2.44 × 10−12 cm3 s−1. The Arrhenius plot of the temperature-dependent rate coefficients yields an activation energy of (−1.99 ± 0.23) kcal mol−1.


 Please wait while we load your content...
Please wait while we load your content...