The Born model can accurately describe electrostatic ion solvation
Abstract
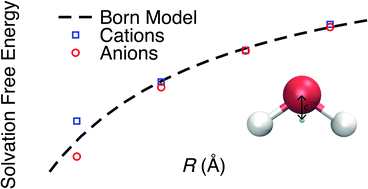
Accurate models of the free energies of ions in solution are crucially important. They can be used to predict and understand the properties of electrolyte solutions in the huge number of important applications where these solutions play a central role such as electrochemical energy storage. The Born model, developed to describe ion solvation free energies, is widely considered to be critically flawed as it predicts a linear response of water to ionic charge, which fails to match water's supposed intrinsic preference to solvate anions over cations. Here, we demonstrate that the asymmetric response observed in simulation is the result of an arbitrary choice of the oxygen atom to be the centre of a water molecule. We show that an alternative and reasonable choice, which places the centre 0.5 Å towards the hydrogen atoms, results in a linear and charge symmetric response of water to ionic charge for a classical water model consistent with the Born model. Therefore, this asymmetry should be regarded as a property of the specific short-range repulsive interaction not an intrinsic electrostatic property of water and so the fact that the Born model does not reproduce it is not a limitation of this approach. We also show that this new water centre results in a more reasonable surface potential contribution to the solvation free energies.


 Please wait while we load your content...
Please wait while we load your content...