Mechanochemical disulfide reduction reveals imprints of noncovalent sulfur⋯oxygen chalcogen bonds in protein-inspired mimics in aqueous solution†
Abstract
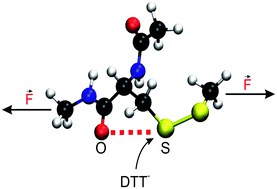
The surprisingly rich chemistry of mechanically activated cleavage of disulfide bonds has been uncovered only recently. Using a disulfide protein mimic together with Cleland's reagent (DTT) as the attacking nucleophile in aqueous solution, our isotensional ab initio simulations add another surprise to the list. They unveil that noncovalent chalcogen–chalcogen 1,5-type S⋯O interactions involving the S–S bridge and γ-carbonyl O are controlling the mechanochemical reactivity of disulfides at very low forces, thus adding a third reactivity regime to the hitherto known ones. In stark contrast to what is found in aqueous solution, no such chalcogen bonding arrangements are observed in the gas phase, which supports the conclusion that water plays a crucial role in stabilizing preferred conformations that support noncovalent S⋯O bonds. These findings open the door to investigate chalcogen bonding in the realm of proteins using single-molecule force spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...