Direct time-resolved detection and quantification of key reactive intermediates in diethyl ether oxidation at T = 450–600 K†
Abstract
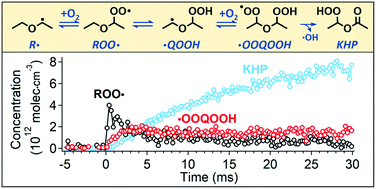
High-pressure multiplexed photoionization mass spectrometry (MPIMS) with tunable vacuum ultraviolet (VUV) ionization radiation from the Lawrence Berkeley Labs Advanced Light Source is used to investigate the oxidation of diethyl ether (DEE). Kinetics and photoionization (PI) spectra are simultaneously measured for the species formed. Several stable products from DEE oxidation are identified and quantified using reference PI cross-sections. In addition, we directly detect and quantify three key chemical intermediates: peroxy (ROO˙), hydroperoxyalkyl peroxy (˙OOQOOH), and ketohydroperoxide (HOOP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, KHP). These intermediates undergo dissociative ionization (DI) into smaller fragments, making their identification by mass spectrometry challenging. With the aid of quantum chemical calculations, we identify the DI channels of these key chemical species and quantify their time-resolved concentrations from the overall carbon atom balance at T = 450 K and P = 7500 torr. This allows the determination of the absolute PI cross-sections of ROO˙, ˙OOQOOH, and KHP into each DI channel directly from experiment. The PI cross-sections in turn enable the quantification of ROO˙, ˙OOQOOH, and KHP from DEE oxidation over a range of experimental conditions that reveal the effects of pressure, O2 concentration, and temperature on the competition among radical decomposition and second O2 addition pathways.
O, KHP). These intermediates undergo dissociative ionization (DI) into smaller fragments, making their identification by mass spectrometry challenging. With the aid of quantum chemical calculations, we identify the DI channels of these key chemical species and quantify their time-resolved concentrations from the overall carbon atom balance at T = 450 K and P = 7500 torr. This allows the determination of the absolute PI cross-sections of ROO˙, ˙OOQOOH, and KHP into each DI channel directly from experiment. The PI cross-sections in turn enable the quantification of ROO˙, ˙OOQOOH, and KHP from DEE oxidation over a range of experimental conditions that reveal the effects of pressure, O2 concentration, and temperature on the competition among radical decomposition and second O2 addition pathways.


 Please wait while we load your content...
Please wait while we load your content...