Nanolubrication in deep eutectic solvents
Abstract
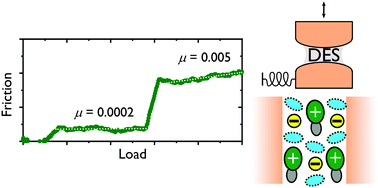
We report surface force balance measurements of the normal surface force and friction between two mica surfaces separated by a nanofilm of the deep eutectic solvent ethaline. Ethaline, a 1 : 2 mixture of choline chloride and ethylene glycol, was studied under dry conditions, under ambient conditions and with added water, revealing surface structural layers and quantised frictional response highly sensitive to water content, including regions of super-lubric behaviour under dry conditions and with added water. We also report exceptionally long-ranged electrostatic repulsion far in excess of that predicted by Debye–Hückel theory for a system with such high electrolyte content, consistent with previously reported observations of “underscreening” in ionic liquid and concentrated aqueous electrolyte systems [Smith et al., J. Phys. Chem. Lett., 2016, 7(12), 2157].
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...