Curvature and size effects hinder halogen bonds with extended π systems†
Abstract
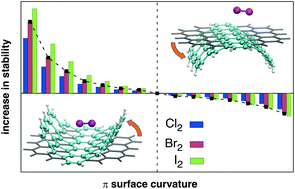
Curvature and size effects in halogen interactions with extended aromatic species have been evaluated, employing computational methods, in dimers formed by dihalogens Cl2, Br2 and I2 with both planar (coronene and circumcoronene) and curved (corannulene, sumanene and C60) aromatic systems. The main controlling factor in these interactions is dispersion, so they become stronger as the size of the halogen grows. The nature of the interaction with the halogen changes depending on the curvature and the extension of the aromatic system. As the aromatic species becomes larger, parallel stacked structures are favoured by dispersion increases over halogen bonded ones. Parallel dimers by the concave side are also favoured as the curvature of the aromatic species increases, while the effect is the opposite by the convex side. Overall, halogen bond interactions are not favoured for large planar or curved aromatic systems; only by the convex face of the most curved structures the dispersion contribution decreases enough so as to make halogen bonded structures competitive with parallel stacked ones.


 Please wait while we load your content...
Please wait while we load your content...