Sulfur doped Li1.3Al0.3Ti1.7(PO4)3 solid electrolytes with enhanced ionic conductivity and a reduced activation energy barrier†
Abstract
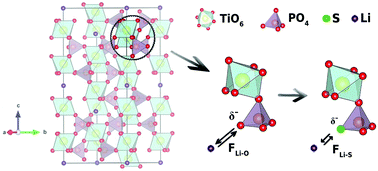
Recently, tailored synthesis of solid electrolytes satisfy multiple challenges, i.e. high ionic conductivity and wide (electro)chemical stability window is of great interest. Although both oxide- and sulfide-based solid electrolytes have distinguished merits for meeting such concerns separately, a new solid electrolyte having the excellent aspects of both materials is pursued. Herein, we report the synthesis of a sulfur-doped Li1.3Al0.3Ti1.7(PO4)3 (LATP) solid electrolyte with a NASICON crystal structure that combines elevated ionic conductivity with intrinsic stability against an ambient atmosphere. Sulfur doping was carried out using sulfur-amine chemistry and the system was characterized by XRD, Raman, XPS, ICP-OES, and EDS analyses. Bader charge analysis was carried out with the aid of density functional theory calculations to characterize charge accumulation in the local environment of the bare and sulfur doped LATP structures. Our results indicate that the partial replacement of oxygen with sulfur yields higher ionic conductivity due to the lower electronegativity of sulfur compared to oxygen, which reduces the attraction of lithium ions. The enhanced ionic conductivity of LATP is attributed to a decreased lithium ion diffusion activation energy barrier upon sulfur doping. Compared to bare LATP, the as-prepared sulfur doped LATP powders were shown to decrease the activation energy barrier by 10.1%. Moreover, an ionic conductivity of 5.21 × 10−4 S cm−1 was obtained for the sulfur doped LATP powders, whereas bare LATP had an ionic conductivity of 1.02 × 10−4 S cm−1 at 40 °C.


 Please wait while we load your content...
Please wait while we load your content...