The behavior of conductivity dynamic modulus and its connection to thermodynamic bulk modulus in ionic liquids†
Abstract
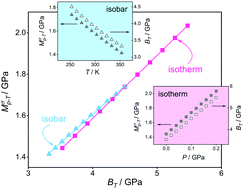
In this paper, we investigate the interplay between the dynamics and thermodynamics of aprotic ionic liquids in the supercooled and normal liquid states. For this purpose, the conductivity dynamic modulus Mσp–T, being defined as the ratio of activation energy (Ep) and activation volume (VT), and its relation to bulk modulus BT under isobaric and isothermal conditions is examined. We found that both isobaric cooling and isothermal compression lead to an increase in Mσp–T. Specifically, Mσp–T(P)T rises linearly similar to BT. Consequently, a direct linear relationship between Mσp–T(P)T and BT is established under isothermal conditions.


 Please wait while we load your content...
Please wait while we load your content...