Local and macrocyclic (anti)aromaticity of porphyrinoids revealed by the topology of the induced magnetic field†
Abstract
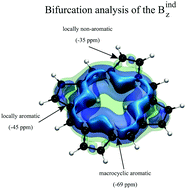
The aromaticity in porphyrinoids results from the π conjugation through two different annular perimeters: the macrocyclic ring and the local heterocyclic rings appended to it. Analyses, based on aromatic stabilization energies (ASE), indicate that the local circuits (6π) are responsible for the significant aromatic stabilization of these systems. This local aromaticity can be coupled with the one from 4n + 2π macrocyclic circuit. It can either compensate for the destabilization due to a 4n π macrocyclic circuit, or be the only source of aromatic stabilization in porphyrinoids with macrocycles without π-conjugated bonds. This “multifaceted” aromatic character of porphyrinoids makes it challenging to analyze their aromaticity using magnetic descriptors because of the intricate interaction of local versus macro-cyclic circulation. In this contribution, we show that the analysis of the bifurcation of the induced magnetic field, Bind, allows clear identification and quantification of both local, and macrocyclic aromaticity, in a representative group of porphyrinioids. In porphyrin, bifurcation values accurately predict the local and macrocyclic contribution rate to overall aromatic stabilization determined by ASE.


 Please wait while we load your content...
Please wait while we load your content...