Tube to ribbon transition in a self-assembling model peptide system
Abstract
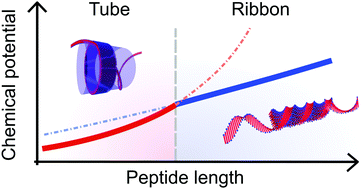
Peptides that self-assemble into β-sheet rich aggregates are known to form a large variety of supramolecular shapes, such as ribbons, tubes or sheets. However, the underlying thermodynamic driving forces for such different structures are still not fully understood, limiting their potential applications. In the AnK peptide system (A = alanine, K = lysine), a structural transition from tubes to ribbons has been shown to occur upon an increase of the peptide length, n, from 6 to 8. In this work we analyze this transition by means of a simple thermodynamic model. We consider three energy contributions to the total free energy: an interfacial tension, a penalty for deviating from the optimal β-sheet twist angle, and a hydrogen bond deformation when the β-sheets adopt a specific self-assembled structure. Whilst the first two contributions merely provide similar constant energy offsets, the hydrogen bond deformations differ depending on the studied structure. Consequently, the tube structure is thermodynamically favored for shorter AnK peptides, with a crossover at n ≈ 13. This qualitative agreement of the model with the experimental observations shows, that we have achieved a good understanding of the underlying thermodynamic features within the self-assembling AnK system.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...