Unique phase behavior of a room-temperature ionic liquid, trimethylpropylammonium bis(fluorosulfonyl)amide: surface melting and its crystallization†
Abstract
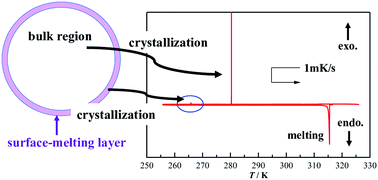
The phase behavior of a representative ammonium-based ionic liquid, trimethylpropylammonium bis(fluorosulfonyl)amide ([N1113][FSA]), was investigated using a laboratory-made differential scanning calorimeter (DSC). The apparatus possesses extremely high sensitivities with stability of ±2 nW in thermal flux and ±1 mK in temperature and a very slow scanning rate of 0.001 mK s−1 in the slowest scanning speed. Besides two ordinary signals from crystallization and melting, a very weak exothermic peak, 1/1000 times that of the main crystallization peak, was observed during the cooling process. The peak was assigned to the crystallization of the surface-melting layer. Both the normal and novel crystallizations occurred during the structural relaxation process. The thickness of the surface-melting layer was estimated to be roughly 70–200 nm. To study the details of the melting processes, DSC experiments were performed with very slow scanning rates (0.02 and 0.03 mK s−1). Two novel endothermic peaks were found in the usual melting trace for the sample with the surface crystallization, and no unusual peaks were observed in the sample without the surface crystallization. We believe that the structure of the surface crystallization phase is different from that of the bulk crystalline phase.


 Please wait while we load your content...
Please wait while we load your content...