An ab initio investigation of the adsorption properties of water on binary AlSi clusters†
Abstract
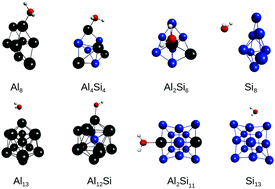
The potential of doped aluminium clusters as catalysts for the water splitting reaction has attracted considerable scientific effort, however, the water–cluster interactions, which are a key step in the overall mechanism, are not fully understood. Here, we report an ab initio investigation of water adsorption on AlSi clusters at the MP2 level to elucidate the bonding and structural properties employing unary and binary 8- and 13-atom clusters, namely, Si8, Al2Si6, Al4Si4, Al8, Si13, Al2Si11, Al12Si, and Al13, which were selected by their relevance and energetic stability. We found that H2O binds via the O atom near to the on-top sites of the Si or Al atoms; in particular, there is a strong preference for the Al sites on the binary AlSi clusters, which is supported by the strong adsorption energy. Furthermore, we found a large enhancement of the adsorption energy on the Al2Si6 and Al2Si11 clusters, which can be explained by the cationic character of the Al site, which increases the Coulomb contribution to the Al+–O− interaction.


 Please wait while we load your content...
Please wait while we load your content...