Effect of chemical aging of aqueous organic aerosols on the rate of their steady-state nucleation†
Abstract
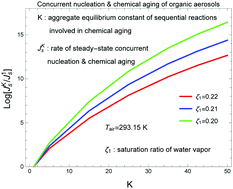
We present the steady-state solution of the kinetic equation for the size and composition distribution of an ensemble of aqueous organic droplets, evolving via nucleation and concomitant chemical aging. The partial differential equation of second order for the temporal evolution of this distribution can be reduced to the canonical form of the multidimensional Fokker-Planck equation, which can be solved analytically by using the method of complete separation of variables. Its solution for the steady-state process provides the stationary distribution of droplets in the vicinity of the saddle point of the free-energy surface as well as the stationary nucleation rate in the form of the product “kinetic (Zeldovich) factor × normalization factor × exp(-free energy of nucleus formation)”. Our numerical evaluations for the formation of aqueous organic aerosols in the air containing the vapors of water, 2-methylglyceric acid, and 3-methyl-4 -hydroxy-benzoic acid, as well as typical atmospheric gaseous species, indicate that the steady-state nucleation rate of such aerosols can be significantly enhanced by their concomitant chemical aging. Thus, one can expect that the application of our approach to the formation and evolution of atmospheric aqueous organic aerosols (via concurrent nucleation and chemical aging) will make aerosol models more adequate and may, once implemented in climate models, improve their forecasting accuracy.


 Please wait while we load your content...
Please wait while we load your content...