Conformational analysis and molecular dynamics of glass-forming aromatic thiacrown ethers†
Abstract
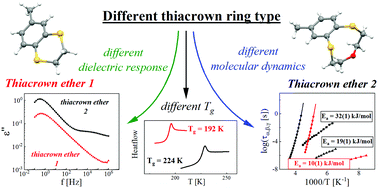
In this work, we report the synthesis, unexpected glass-forming properties, molecular dynamics and conformational analysis of two thiacrown ethers: 6-methyl-2,3-dihydro-1,4-benzodithiine (1), with a six-membered heterocyclic ring, and macrocyclic 2,3-(4′-methylbenzo)-1,4-dithia-7-oxacyclononane (2). Based on the calorimetric studies, we showed that compound 1 is a viscous liquid at room temperature undergoing vitrification at 192 K. Compound 2 is a crystalline solid at room temperature characterized by a melting point at 331 K; however, it can be vitrified with ease after being melted by cooling down to 224 K. This gave us the unique possibility to analyze the dielectric response and to follow the molecular dynamics in supercooled liquid and glassy states for each thiacrown ether. Two relaxation processes were found for compound 1, which are structural α-relaxation, connected with the collective rotational motions of molecules in a liquid, and a low-temperature secondary γ-process, resulting from conformational changes in the heterocyclic ring. Beside these two relaxation processes, an additional intermolecular β-process of JG type was detected in the case of compound 2. Finally, based on the analysis of the thermal evolution of the Kirkwood–Fröhlich factor, it has also been shown that thiacrown ethers may be characterized by a local ordering between neighboring molecules in the supercooled liquid state.


 Please wait while we load your content...
Please wait while we load your content...