Electron spin relaxation properties of atomic hydrogen encapsulated in octavinyl POSS cages
Abstract
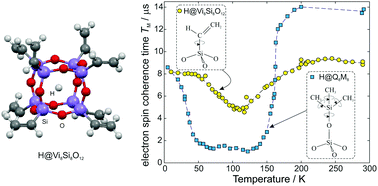
The electron spin relaxation times of encapsulated atomic hydrogen in the vinyl derivative of silsesquioxane (R8Si8O12) cages (R = CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) are studied in detail by pulse electron paramagnetic resonance (EPR) methods in the temperature range between 10 and 300 K. The temperature dependence of the spin–lattice relaxation time, T1, shows similar behaviour with previously studied derivatives that typically involve Raman and thermally activated processes. The room-temperature phase-memory time TM = 9 μs is comparable to those reported for different alkyl derivatives and exhibits a characteristic temperature dependence with a considerable reduction below 200 K as a result of dynamic effects like methyl group rotation. However, this reduction is modest for the vinyl derivative since the minimum observed TM = 5 μs is much longer than the value of 1 μs reported for methyl-containing derivatives like R = C2H5, C3H7 (n-propyl), or OSi(CH3)2H. This discrepancy is attributed to the different rotation dynamics of the vinyl group, as evidenced by the determined activation energy and rotation frequency.
CH2) are studied in detail by pulse electron paramagnetic resonance (EPR) methods in the temperature range between 10 and 300 K. The temperature dependence of the spin–lattice relaxation time, T1, shows similar behaviour with previously studied derivatives that typically involve Raman and thermally activated processes. The room-temperature phase-memory time TM = 9 μs is comparable to those reported for different alkyl derivatives and exhibits a characteristic temperature dependence with a considerable reduction below 200 K as a result of dynamic effects like methyl group rotation. However, this reduction is modest for the vinyl derivative since the minimum observed TM = 5 μs is much longer than the value of 1 μs reported for methyl-containing derivatives like R = C2H5, C3H7 (n-propyl), or OSi(CH3)2H. This discrepancy is attributed to the different rotation dynamics of the vinyl group, as evidenced by the determined activation energy and rotation frequency.


 Please wait while we load your content...
Please wait while we load your content...