The role of the iodine-atom adduct in the synthesis and kinetics of methyl vinyl ketone oxide—a resonance-stabilized Criegee intermediate†
Abstract
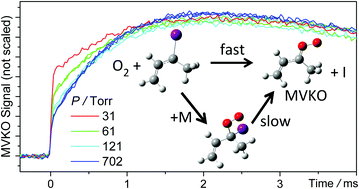
Isoprene is the most abundant alkene in the atmosphere. Ozonolysis of isoprene produces three very reactive carbonyl oxides (Criegee intermediates), including formaldehyde oxide, methyl vinyl ketone oxide (MVKO, CH3(C2H3)COO), and methacrolein oxide. The latter two Criegee intermediates are resonance-stabilized due to the vinyl group. Recently, the electronic spectrum of thermalized MVKO has been reported [Caravan, et al., Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 9733]. In this work, we utilized this strong UV/visible absorption to investigate the reaction kinetics of MVKO with SO2 under a wide pressure range of 4 to 700 Torr. We followed a new method [Barber, et al., J. Am. Chem. Soc., 2018, 140, 10866], in which MVKO is produced through the reaction of a resonance-stabilized iodoalkene radical with O2. The experimental data are consistent with a kinetic model that the reaction goes through an adduct of CH3(C2H3)CIOO, similar to the cases of H/alkyl substituted Criegee intermediates. However, different from the H/alkyl adducts, which are stable over the time scales of typical kinetic experiments, this vinyl adduct CH3(C2H3)CIOO is less stable and decomposes to MVKO + I at a time scale of 10−3 s (faster at higher temperature), consistent with the results of quantum chemistry calculations and the fact that the resonance stabilization is disrupted at the adduct structure. The adduct decomposition is the major pathway that forms MVKO for pressures higher than 50 Torr. In addition, temperature dependence has been investigated for 278–319 K. The experimental activation energy of the adduct decomposition was measured to be 12.7 ± 0.3 kcal mol−1, consistent with the calculated dissociation energy of the adduct to MVKO + I (14 kcal mol−1). Furthermore, the temperature dependent rate coefficient of MVKO + SO2 reaction has been measured to be kSO2 = (4.0 ± 0.6) × 10−11 cm3 s−1 at 4–700 Torr and 298 K with a negative activation energy of −3.7 ± 0.4 kcal mol−1.


 Please wait while we load your content...
Please wait while we load your content...