Conjugated bilayer structure of the homogeneous solid–liquid interface of metals†
Abstract
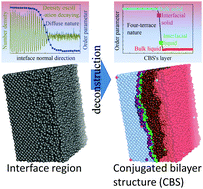
The concept of “interfacial region” has long prevailed for over half century for describing the homogeneous solid–liquid (SL) interface of metals, but its intrinsic structure is still unclear due to the homogeneity. In this study, we reveal, for the first time, the intrinsic structure of these homogeneous SL interfaces consisting of two conjugated monoatomic layers of interfacial solid (IS) and interfacial liquid (IL) with a certain degree of corrugation via molecular dynamics simulations. We named it as the conjugated bilayer structure (CBS). In the framework of CBS, only the IS + IL bilayer plays stepwise transition roles from the solid to the liquid, which defines the four-terrace nature of the interface and act simultaneously as the boundaries of the bilateral bulk phases. The inherent diffuse nature of the “interfacial region” is proven originating from the corrugation of the IS + IL bilayer and its four-terrace nature. More importantly, the CBS also explains that the interfacial free energy originates mainly from the increase in the potential energy of the IS layer relative to its counterpart bulk solid instead of the previously argued entropy loss of the liquid phase. After all these verifications and interpretations, the CBS was verified as the intrinsic structure of the homogeneous SL interface of metals. Accordingly, we argue that the concept of CBS also resolves the volume-bearing flaw of the “interfacial region” concept and can definitively locate the intrinsic surface according to the capillary wave theory.


 Please wait while we load your content...
Please wait while we load your content...