Unprecedented stability enhancement of multiply charged anions through decoration with negative electron affinity noble gases†
Abstract
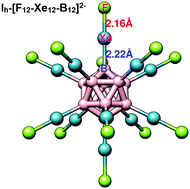
The present communication reports unprecedented stabilization of multiply charged anion, B12F122−, through insertion of noble gas (Ng) atoms possessing negative electron affinity into B–F bonds, resulting in the formation of stable icosahedral B12Ng12F122−, where the HOMO is stabilized significantly and the binding energy of the second excess electron is increased remarkably. Unprecedented stability enhancement with Ng is attributed to a strong covalent B–Ng bond, increased charge delocalization and increased electrostatic interaction between the oppositely charged centers.


 Please wait while we load your content...
Please wait while we load your content...