Thermodynamics of mixing methanol with supercritical CO2 as seen from computer simulations and thermodynamic integration
Abstract
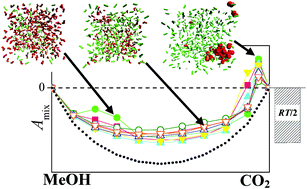
The changes in extensive thermodynamic quantities, such as volume, energy, Helmholtz free energy and entropy, occurring upon mixing liquid methanol with supercritical CO2, are calculated using Monte Carlo simulations and thermodynamic integration for all eight combinations of four methanol and two CO2 potential models in the entire composition range at 313 K. The obtained results are also compared with experimental data whenever possible. The transition of the system from liquid to a supercritical state is found to occur at this temperature around a CO2 mole fraction value of 0.95 with all model combinations considered. This liquid to supercritical transition is always accompanied by positive Helmholtz free energy of mixing values and, consequently, by the non-miscibility of the two components. Furthermore, both this non-miscibility around the liquid to supercritical transition and also the miscibility of the two components below this transition, in the liquid regime, are found to be primarily of the energetic rather than entropic origin; the entropy of mixing turns out to be very close to zero, and around the liquid to supercritical transition even its qualitative behaviour is strongly model dependent. Finally, it is found that the methanol expansion coefficient is not sensitive to the details of the potential models, and it is always in excellent agreement with the experimental data. On the other hand, both the volume and the energy of mixing depend strongly on the molar volume of neat CO2 in the model being used, and in this respect the TraPPE model of CO2 [J. J. Potoff and J. I. Siepmann, AIChE J., 2001, 47, 1676] performs considerably better than that of Zhang and Duan [Z. Zhang and Z. Duan, J. Chem. Phys., 2005, 122, 214507].


 Please wait while we load your content...
Please wait while we load your content...