Understanding ring-closing and racemization to prepare α-amino acid NCA and NTA monomers: a DFT study†
Abstract
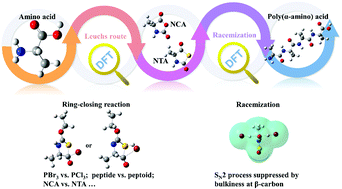
Polypeptides and polypeptoids are promising materials in biomedical applications bearing α-amino acid repeating units, which are prepared from ring-opening polymerizations of α-amino acid N-carboxyanhydride (NCA) or N-thiocarboxyanydride (NTA) monomers. Detailed studies on monomer synthetic routes are essential to explore new α-amino acid NCA and NTA monomers as well as the corresponding poly(α-amino acid) materials. In this contribution, density functional theory (DFT) is applied to investigate the mechanism of the Leuchs approach including two possible pathways, precursor structure and racemization in the ring-closing reaction. According to DFT calculations, pathway 2 is preferred with lower ΔG than pathway 1, and the rate-determining step is recognized as an SN2 substitution with releasing equivalent halogenated hydrocarbon, which explains our experimental observations. Racemization results from the reaction between the NTA monomer and a strong protonic acid, which can be suppressed by low temperature and short reaction time. Racemization is inhibited by steric hindrance in those NTAs of α-amino acids containing high bulkiness at the β-carbon, such as leucine-NTA.


 Please wait while we load your content...
Please wait while we load your content...