Photon-mediated charge exchange reactions between 39K atoms and 40Ca+ ions in a hybrid trap†
Abstract
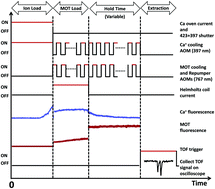
We present experimental evidence of charge exchange between laser-cooled potassium 39K atoms and calcium 40Ca+ ions in a hybrid atom–ion trap and give quantitative theoretical explanations for the observations. The 39K atoms and 40Ca+ ions are held in a magneto-optical (MOT) and a linear Paul trap, respectively. Fluorescence detection and high resolution time of flight mass spectra for both species are used to determine the remaining number of 40Ca+ ions, the increasing number of 39K+ ions, and 39K number density as functions of time. Simultaneous trap operation is guaranteed by alternating periods of MOT and 40Ca+ cooling lights, thus avoiding direct ionization of 39K by the 40Ca+ cooling light. We show that the K–Ca+ charge-exchange rate coefficient increases linearly from zero with 39K number density and the fraction of 40Ca+ ions in the 4p 2P1/2 electronically-excited state. Combined with our theoretical analysis, we conclude that these data can only be explained by a process that starts with a potassium atom in its electronic ground state and a calcium ion in its excited 4p 2P1/2 state producing ground-state 39K+ ions and metastable, neutral Ca (3d4p 3P1) atoms, releasing only 150 cm−1 equivalent relative kinetic energy. Charge-exchange between either ground- or excited-state 39K and ground-state 40Ca+ is negligibly small as no energetically-favorable product states are available. Our experimental and theoretical rate coefficients are in agreement given the uncertainty budgets.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...