The origin of the hysteresis in cyclic voltammetric response of alkaline methanol electrooxidation†
Abstract
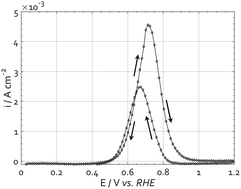
The mechanism of the alkaline methanol electrooxidation reaction on platinum is complex and not fully understood. However, a better understanding will facilitate reaching the theoretical performance of an alkaline methanol fuel cell. Cyclic voltammetry is an often used method to investigate the mechanism of electrochemical reactions. The cyclic voltammogram of methanol electrooxidation shows a hysteresis in potential between the oxidation peaks in the forward and backward scans. The origin of this hysteresis has not been fully clarified. By means of parameter variations, such as the upper potential or the starting point of the CV measurements, and by physicochemical modeling, we investigate the formation of platinum oxides as a possible cause of this behaviour. The experiments show that the formation of platinum oxides is more likely to cause the hysteresis than the strongly adsorbed intermediates, which are formed during the forward scan. The simulation includes the formation of platinum oxide species and supports the experimental results that the hysteresis is due to the formation and reduction of platinum oxides. Besides, the simulation reveals that in the investigated potential area, different oxide forms are present.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...