Degradation of the transition metal@Pt core–shell nanoparticle catalyst: a DFT study†
Abstract
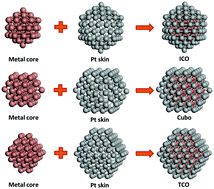
Electrocatalysts in acidic media face the issues of inactivation and degradation with complex thermodynamic processes. A density functional theory (DFT) calculation is performed to investigate the galvanic and pitting etching processes of metal@Pt (M@Pt) core−shell nanoparticles (12 transition elements are selected to replace the core atoms). The dissolution process with atomic etching follows the dissolution potential site-dependence phenomena, and the dissolution potential of the Pt shell exhibits a negative linear correlation with the average d-band center of the Pt shell. We have found that the specific shape effect, core−shell contact area and period effect all affect the potential difference at each step in the dissolution process. Meanwhile, the core atom segregation reduces the dissolution potential to form defects on the outermost shell, which is the driving force of halogen-pitting. By analyzing the 12 core elements and M@Pt nanoparticles of three specific shapes, Ir@Pt nanoparticles with a TCO-structure exhibit a high initial potential in multistep dissolution process throughout the galvanic etching process and good performance with respect to pitting corrosion and are strong candidates for nanoparticle catalysts.


 Please wait while we load your content...
Please wait while we load your content...