Four resonance structures elucidate double-bond isomerisation of a biological chromophore†
Abstract
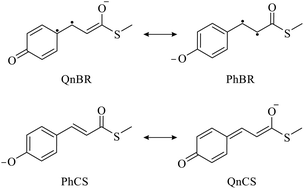
Photoinduced double-bond isomerisation of the chromophore of photoactive yellow protein (PYP) is highly sensitive to chromophore–protein interactions. On the basis of high-level ab initio calculations, we scrutinise the effect of hydrogen bonds on the photophysical and photochemical properties of the chromophore. We identify four resonance structures – two closed-shell and two biradicaloid – that elucidate the electronic structure of the ground and first excited states involved in the isomerisation process. Changing the relative energies of the resonance structures by hydrogen-bonding interactions tunes all photochemical properties of the chromophore in an interdependent manner. Our study sheds new light on the role of the chromophore electronic structure in tuning in photosensors and fluorescent proteins.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...