Complexation of Pluronic L62 (EO6)–(PO34)–(EO6)/aerosol-OT (sodium bis(2-ethylhexyl)sulfosuccinate) in aqueous solutions investigated by small angle neutron scattering†‡
Abstract
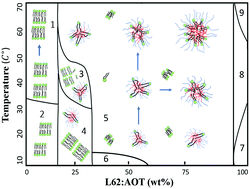
We investigate the phase behaviours of Pluronic L62 in aqueous solution in the presence of aerosol-OT (AOT) molecules by small angle neutron scattering (SANS). The presence of AOT significantly changes the micellization phenomenon of L62 micelles in aqueous solution, including their critical micelle temperature (CMT), global size, and asphericity. The origin of these observations is attributed to the complexation between the neutral L62 surfactants and the ionic AOT molecules, which additionally provides charge to the mixed micelles: we analyse the data and extract meaningful information using the Ornstein–Zernike integral formalism. As a result, we observe that the co-micellization of L62 and AOT is very stable across a wide temperature range.


 Please wait while we load your content...
Please wait while we load your content...