Reaction mechanism, energetics, and kinetics of the water-assisted thioformaldehyde + ˙OH reaction and the fate of its product radical under tropospheric conditions†
Abstract
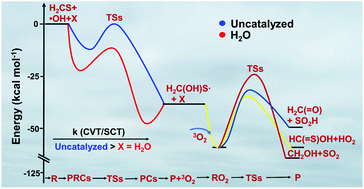
The reactions of thioformaldehyde (H2CS) with OH radicals and assisted by a single water molecule have been investigated using high level ab initio quantum chemistry calculations. The H2CS + ˙OH reaction can in principle proceed through: (1) abstraction, and (2) addition pathways. The barrier height for the addition reaction in the absence of a catalyst was found to be −0.8 kcal mol−1, relative to the separated reactants, which has a ∼1.0 kcal mol−1 lower barrier than the abstraction channel. The H2CS + ˙OH reaction assisted by a single water molecule reduces the barrier heights significantly for both the addition and abstraction channels, to −5.5 and −6.7 kcal mol−1 respectively, compared to the un-catalyzed H2CS + ˙OH reaction. These values suggest that water lowers the barriers by ∼6.0 kcal mol−1 for both reaction paths. The rate constants for the H2CS⋯H2O + ˙OH and OH⋯H2O + H2CS bimolecular reaction channels were calculated using Canonical Variational Transition state theory (CVT) in conjunction with the Small Curvature Tunneling (SCT) method over the atmospherically relevant temperatures between 200 and 400 K. Rate constants for the H2CS + ˙OH reaction paths for comparison with the H2CS + ˙OH + H2O reaction in the same temperature range were also computed. The results suggest that the rate of the H2CS + ˙OH + H2O reaction is slower than that of the H2CS + ˙OH reaction by ∼1–4 orders of magnitude in the temperatures between 200 and 400 K. For example, at 300 K, the rates of the H2CS + ˙OH + H2O and H2CS + ˙OH reactions were found to be 2.2 × 10−8 s−1 and 6.4 × 10−6 s−1, respectively, calculated using [OH] = 1.0 × 106 molecules cm−3, and [H2O] = 8.2 × 1017 molecules cm−3 (300 K, RH 100%) atmospheric conditions. Electronic structure calculations on the H2C(OH)S˙ product in the presence of 3O2 were also performed. The results show that H2CS is removed from the atmosphere primarily by reacting with ˙OH and O2 to form thioformic acid, HO2, formaldehyde, and SO2 as the main end products.


 Please wait while we load your content...
Please wait while we load your content...