A comprehensive study of the thermal conductivity of the hard sphere fluid and solid by molecular dynamics simulation
Abstract
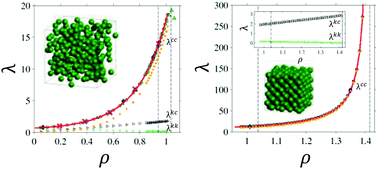
This work reports a new set of hard sphere (HS) thermal conductivity coefficient, λ, data obtained by Molecular Dynamics (MD) computer simulation, over a density range covering the dilute fluid to near the close-packed solid, and for a large number of particles (up to N = 13 1072) and long simulation times. The N-dependence of the thermal conductivity is shown to be proportional to N−2/3 to a good approximation over a wide range of system sizes, which enabled λ values in the thermodynamic limit to be predicted accurately. The fluid and solid λ can be represented well by the Enskog theory (ET) formula, λE, times a density-dependent correction term, which is close to unity for the fluid and practically constant for the solid. The convergence of the MD λ data back towards ET in the metastable fluid starts just above the freezing density. For the HS solid and dense fluid it was found that the thermal conductivity is nearly linear in pressure, as has been observed experimentally for a number of solids. Simple excess entropy scaling over the higher density fluid phase region was found, and Rosenfeld's exponential relationship can be fitted to the simulation data for the solid to a high degree of accuracy. The simulation analysis has revealed a number of new trends in the behaviour of the HS thermal conductivity which could be useful in building more accurate models for heat conduction in experimental systems.


 Please wait while we load your content...
Please wait while we load your content...