A multiporous carbon family with superior stability, tunable electronic structures and amazing hydrogen storage capability†
Abstract
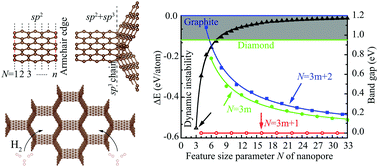
The traditional view that natural allotropes are more stable than artificially synthesized structures is widely accepted. For instance, graphite and diamond are more energetically favorable than other new carbon allotropes no matter whether they are experimentally prepared or theoretically predicted. Surprisingly, we find that a family of multiporous carbon (N-diaphenes) could be thermodynamically more stable than natural diamond with the increase of its feature size parameter N. Multiporous N-diaphenes exhibit extremely strong anisotropic mechanical properties and their ideal strength linearly depends on the corresponding yield strain. Density functional theory (DFT) calculations reveal that the bandgap hierarchy of N-diaphenes is inherited from their precursors. In addition, N-diaphenes exhibit superior capability for hydrogen storage due to their large specific surface areas.


 Please wait while we load your content...
Please wait while we load your content...