Anomalous protein kinetics on low-fouling surfaces†
Abstract
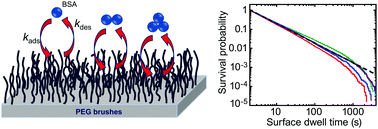
In this work, protein–surface interactions were probed in terms of adsorption and desorption of a model protein, bovine serum albumin, on a low-fouling surface with single-molecule localization microscopy. Single-molecule experiments enable precise determination of both adsorption and desorption rates. Strikingly the experimental data show anomalous desorption kinetics, evident as a surface dwell time that exhibits a power-law distribution, i.e. a heavy-tailed rather than the expected exponential distribution. As a direct consequence of this heavy-tailed distribution, the average desorption rate depends upon the time scale of the experiment and the protein surface concentration does not reach equilibrium. Further analysis reveals that the observed anomalous desorption emerges due to the reversible formation of a small fraction of soluble protein multimers (small oligomers), such that each one desorbs from the surface with a different rate. The overall kinetics can be described by a series of elementary reactions, yielding simple scaling relations that predict experimental observations. This work reveals a mechanistic origin for anomalous desorption kinetics that can be employed to interpret observations where low-protein fouling surfaces eventually foul when in long-term contact with protein solutions. The work also provides new insights that can be used to define design principles for non-fouling surfaces and to predict their performance.


 Please wait while we load your content...
Please wait while we load your content...