Hyaluronan biopolymers release water upon pH-induced gelation†
Abstract
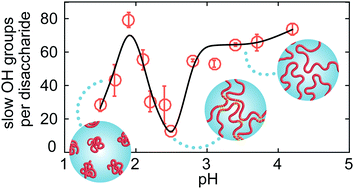
We study the relation between the macroscopic viscoelastic properties of aqueous hyaluronan polymer solutions and the molecular-scale dynamics of water using rheology measurements, differential dynamic microscopy, and polarization-resolved infrared pump–probe spectroscopy. We observe that the addition of hyaluronan to water leads to a slowing down of the reorientation of a fraction of the water molecules. Near pH 2.4, the viscosity of the hyaluronan solution reaches a maximum, while the number of slowed down water molecules reaches a minimum. This implies that the water molecules become on average more mobile when the solution becomes more viscous. This observation indicates that the increase in viscosity involves the expulsion of hydration water from the surfaces of the hyaluronan polymers, and a bundling of the hyaluronan polymer chains.


 Please wait while we load your content...
Please wait while we load your content...