Incorporation of trivalent cations in NaX zeolite nanocrystals for the adsorption of O2 in the presence of CO2†
Abstract
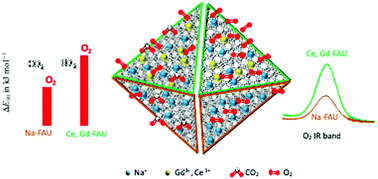
The O2 and CO2 sorption properties of nanosized zeolite X with faujasite type structure through a partial ionic exchange of sodium (Na+) by trivalent cations (Gd3+ and Ce3+) were evaluated. Three faujasite samples were studied, the as-synthesized Na–X possessing Na+ solely, and the modified samples Na–Gd–X and Na–Ce–X containing Gd3+ (1.8 wt%) and Ce3+ (0.82 wt%), respectively. Incorporating scarce amounts of trivalent cations modified the adsorption affinity of zeolites towards O2 and CO2 as demonstrated by in situ Fourier-transform infrared spectroscopy (FTIR). While Na–Ce–X encounters the highest O2 physisorption capacity, the Na–Gd–X is adsorbing the highest quantities of molecular CO2. All three samples exhibit the chemisorbed CO2 in the form of carbonates, while the Na–X stores carbonates in monodentate and polydentate forms, the Na–Gd–X and Na–Ce–X allow the formation of polydentate carbonates only. Density functional theory (DFT) calculations revealed that trivalent cations tend to adsorb gases through two cations simultaneously which explains the presence of polydentate carbonates exclusively in the corresponding modified zeolites. The DFT results confirmed the higher affinity of Na–Gd–X and Na–Ce–X nanocrystals towards O2 in the presence of CO2. The affinity of Na–Gd–X and Na–Ce–X nanocrystals towards O2 opens the door of their use as oxygen transporters for medical applications where CO2 is constantly present. The toxicity of the nanosized zeolites and their performance in O2 release are reported too.


 Please wait while we load your content...
Please wait while we load your content...