Influence of an external electric field on the deprotonation reactions of an Fe3+-solvated molecule: a reactive molecular dynamics study
Abstract
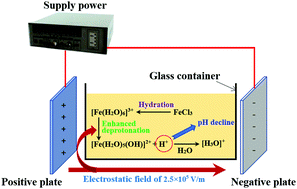
The influence of an external electric field (EEF) on the deprotonation reaction of Fe3+-solvated molecules was studied using reactive molecular dynamics (ReaxFF MD) simulations. It was examined in terms of changes in structural properties, kinetics, system energy, and reaction products under an EEF, and the results were further verified experimentally. The research results show that the presence of an EEF will affect the distribution of water molecules around Fe3+ and provide energy for the fracturing of O–H bonds. The increase in the state of reaction products represented by H+ also suggests that the EEF can promote the deprotonation reaction of Fe3+-solvated molecules. The viscosity of the system is significantly increased under an EEF. The experimental results for verification show that the pH of the FeCl3 solution is reduced under the action of an EEF, which means that the hydrolysis of Fe3+ has been promoted. The experimental results are consistent with the results of the MD simulations.

 Please wait while we load your content...
Please wait while we load your content...