Crystallization of highly supercooled glass-forming alloys induced by anomalous surface wetting†
Abstract
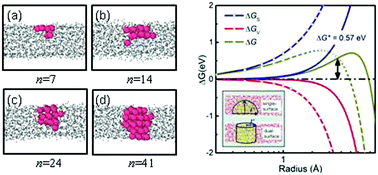
Crystallization in highly supercooled Cu50Zr50 films close to the glass transition is studied by using molecular dynamics simulations. Spontaneous nucleation is observed at the simulation timescale in contrast to the bulk counterpart. We find that nucleation occurs at free surfaces owing to the partial wetting of the nucleus by melt. The anomalous wetting phenomenon is closely related to strong density layering arising from the surface: the high density associated with surface layering increases surface energy of supercooled melts, resulting in that one facet of the crystalline embryo is preferentially formed on the film surface. The surface-based embryo is then developed into a stable nucleus by bridging two surfaces of thin films. The kinetics and thermodynamics analyses based on the mean first-passage time method show that the nucleation process still follows the description of the classical nucleation theory despite extremely high supercoolings. In nucleating, the slow interface dynamics becomes dominant and induces a low nucleation rate although the nucleation barrier is very low. The subsequent crystal growth is found to proceed in a quasi-two-dimensional manner with a ramified interface morphology, which is analogous to percolative crystals predicted in glass-forming liquids.


 Please wait while we load your content...
Please wait while we load your content...